Abstract
Background
Stem cell transplantation (SCT) represents the effective therapeutic option for acute myeloid leukemia (AML). However, recurring relapses remains the major cause of treatment failure. Although limited therapeutic options are available after a relapse of AML following the first autologous/allogeneic SCT (SCT1), the second SCT from the allogeneic donor (allo-SCT2) could achieve durable remission in some selective patients. As a response, this study aimed to identify factors that affect survival outcomes and develop a prognostic model using identified factors for patients who are candidate for allo-SCT2.
Method
A retrospective cohort of consecutive 78 adult AML patients who received allo-SCT2 following any cycles of intensive chemotherapy (IC) for the disease relapse after SCT1 was presented. Twenty-five patients underwent relapse after autologous SCT1, whereas fifty-three patients faced relapsed after allogeneic SCT1.
Result
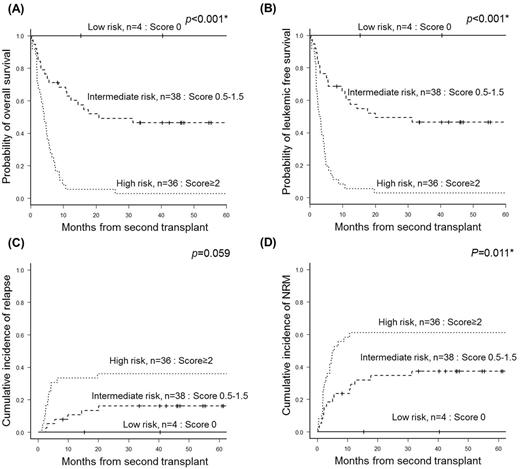
The median age of the patients was 41 years old (19-67). At the time of allo-SCT2, 18 patients were refractory despite administration of IC, but 60 achieved response of complete remission (i.e. CR, n=27)/ CR with incomplete (CRi, n=33); after 1 cycle of IC, 48 achieved response; after 2 cycles, 11; and after 3 cycles, only 1. The median follow-up of survivors (n=22) was 40.7 months (1.8-169.2). Four years from allo-SCT2, the overall survival (OS) rate, leukemic-free survival rate, and the cumulative incidences of relapse and non-relapse mortality were 28.7%, 28.7%, 24.6%, and 43.6%, respectively. In multivariate analysis, the poor cytogenetic risk at diagnosis (p <0.001), the circulating blast ≥ 20% at relapse (p=0.007), the duration from first transplant to relapse < 9 months (p=0.003), the failure to achieve morphologic CR at allo-SCT2 (p=0.001) were potential factors associating poor OS. These factors taken into account, the Catholic AML Second transplantation (CAST) model was developed; each intermediate and poor cytogenetic risk at diagnosis [0.5 and 1 point], peripheral blast≥20% at relapse [1 point], duration from first transplant to relapse <9 months [1 point], and failure to achieve morphological CR at allo-SCT2 [1 point] added up to present a total score from 0 to 4. The CAST model identified three subgroups with 4-year OS of 100.0% in low (score 0, n=4), 46.5% in intermediate (score 0.5-1.5, n=38), and 2.8% in high risk (score≥2, n=36) groups (p <0.001).
Conclusion
The CAST model offers a relevant tool to predict survival for patients receiving allo-SCT2, a fact that may be useful to making an appropriate decision in terms of selecting a candidate for allo-SCT2 in relapsed AML after SCT1.
Kim: Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Il-Yang: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda: Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal